Calcium Chloride and Water Equation
Calcium carbonate can also be an additive to food products for livestock animals and humans and as a supplement in vitamins. Calcium sulfate or calcium sulphate is the inorganic compound with the formula CaSO 4 and related hydratesIn the form of γ-anhydrite the anhydrous form it is used as a desiccantOne particular hydrate is better known as plaster of Paris and another occurs naturally as the mineral gypsumIt has many uses in industry.

Nucleophilic Addition Elimination Acylation A Level Chemistry Tutor Aqa Ocr Edexcel Cie Youtube Tutor Online Tutoring Chemistry
A multistage process for extracting minerals from desalination reject brine while concurrently capturing CO 2 was evaluated.

. The optimum conditions for NaOH CO 2 flowrate and salinity for the process were found to be 5 gL 2 Lmin and 75 gL respectively. It therefore isn. Calcium hydroxide which is a base reacts.
The balanced equation for the reaction. It is also hydrolyzed to cyanamide H 2 NCN. P ΔH ΔQ ΔV.
AGF is implemented in the field using a mobile refrigeration plant which circulates chilled calcium chloride brine through freeze pipes removing heat from the soil and freezing the soils pore water. Structure of Ca 3 PO 4 2. Calcium carbide compound reacts with nitrogen at higher temperatures to produce calcium cyanamide.
Calcium chloride is more effective at melting ice because it can break down into three ions instead of two. Only compounds that are aqueous are split into ions. P 7 41 11.
One calcium ion and two chloride ions. The drying agent used in drying hydrogen chloride gas is conc. Uses of Calcium phosphate Ca 3 PO 4 2.
When alkalinity is reported it is expressed as calcium carbonate or CaCO3-. More ions mean more ions getting in the way of those rigid ice bonds. Distilled water has essentially nothing in it other.
CO 2 uptake and calcium recovery were optimized using Response Surface Methodology. Sodium chloride may impart a salty taste at 250 mgL. Hydrogen chloride gas is highly soluble in water.
Commonly the term called nitrolime which is a calcium cyanamide can be used as fertilizer. Liquid nitrogen may also be used however it is allowed to evaporate into the atmosphere rather than being recirculated. Metallic oxides react with acids to give salts and water similar to the reaction of a base with an acid metallic oxides are said to be basic oxides.
Write the equation and balance it if necessary NaClaq AgNO 3 aq AgCls NaNO 3 aq Step 2. The list of uses of calcium carbonate is given below. Often however cities use calcium chloride CaCl 2 another type of salt on their icy streets.
There are many methods to measure the chloride concentration in water but the normal one is the. In the equation above 50000 represents the equivalent weight of CaCO3 50 multiplied by 1000 mg. The solubility of AgBr in water is only 000013 gram per liter.
The oil will float on the water preventing the air from dissolving. Ag Br- 71 x 10-7 M. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl.
All forms are white solids that are poorly soluble in water. Standards for public drinking water require chloride levels that do not exceed 250 mgL. Calcium phosphate is insoluble in water but soluble in acids.
Oiling can either slow down the process or. In water and sewer treatment plants calcium carbonate is employed in the removal of acidity and impurities. Phosphorous pentoxide and calcium oxide are good drying agents but they cannot be used to dry hydrogen chloride gas because they react with hydrogen chloride.
Taking the square root of both sides of this equation gives the equilibrium concentrations of the Ag and Br-ions. The use of calcium chloride cuts out the water vapour in the air and prevents rusting. It is used in natural farming.
100 removal of Ca. By making considerable change in enthalpy equation we get. Fifty thousand is a constant used in the formula.
If there is any moisture in the air anhydrous calcium chloride will absorb it. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. Pour boiled distilled water into test tube rmB then add about 1rmml of oil and cork it.
Sodium chlorideaq silver nitrateaq silver chlorides sodium nitrateaq Solution. Once we know how many moles of AgBr dissolve in a liter of water we can calculate the solubility in grams per liter. 216 Reaction of a Non-metallic Oxide with Base You saw the reaction between carbon dioxide and calcium hydroxide lime water in Activity 25.
However an online Chemical Equation Balancer Calculator will provide you the balanced equation equilibrium constant with chemical name and formula of all reactants and product of a chemical equation. A number of design considerations must be taken. What if the sample used in the test above was distilled water.
CaC 2 N 2 CaCN 2 C. The most effective prevention tool is galvanization which prevents water and air from reacting with the metal substance. It is represented by using the below equation.
Calcium phosphate may dissolve slightly in CO 2-containing water. However magnesium or calcium chloride are generally not detected by taste until reaching levels of 1000 mgL. Sodium chloride is the salt.
Write the ionic equation for the word equation. Water soluble calcium phosphate is an important material for plant growth and is commonly dispersed in the soil. Place some anhydrous calcium chloride in test tube rmC and cork it.

Pin By Carin Barber On Chemistry Notes Chemistry Notes Chemistry Math

Ncertexemplarsolutionsforclass10science Class10sciencencertexemplarclass10science Learninsta Solutions Science Chapter

Solubility Product Constants Silver Chloride Agcl Is Rather Insoluble In Water Careful Experiments Show That If Solid Solubility Experiments Silver Chloride
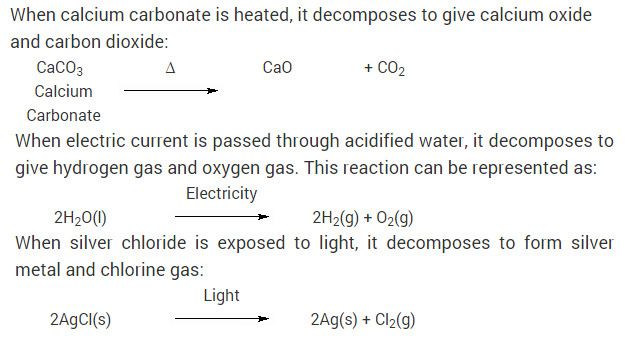
Chemical Reactions And Equation Cbse Class 10 Science Ncert Solutions Chemical Reactions Science Textbook Science

Calcium Chloride Baking Soda Water Reaction Calcium Chloride Baking Soda Baking

Chemical Reactions And Equation Cbse Class 10 Science Ncert Solutions Maths Ncert Solutions Science Textbook Chemical Reactions
0 Response to "Calcium Chloride and Water Equation"
Post a Comment